In this article, we are going to know about diatomic elements. But first, let us get a brief introduction of what molecules are. So, Molecules are the smallest working unit of a substance. They have all the properties and take part in chemical reactions. They can be composed of one or more atoms. Therefore, atoms form chemical bonds with each other. This is how molecules are constructed. Now, let’s see how many atoms can form a bond together. However, based on atoms that form a chemical bond, there can be three types of molecules. These are mono-atomic, di-atomic, and polyatomic molecules. In this article, we are going to discuss di-atomic elements. So, what are di-atomic elements? What are the examples, and formulas, and how to remember them easily? So first, take a look at the definition.
Diatomic Element Definition
When we study the structure of the earth, we see there are major components that make up the earth’s atmosphere. These are elements like nitrogen, oxygen, a little amount of argon, and some other gases. Now, take a look at the distribution of these gases in the atmosphere. You will find that the atmosphere is composed of almost 78% nitrogen and 21% of oxygen. So, this oxygen, O2, and nitrogen, N2, are part of this element group. But why are these called so?
You will find the answer in the word itself. Take a look at the prefix ‘di-‘ in the word. It is a Greek word that means ‘two”. So, when we say a molecule is diatomic, we mean that atom cannot exist by itself. There has to be a bond of at least two atoms present. You will never find lone O atoms floating around the atmosphere. Oxygen exists as O2 because the O atoms must live in pairs. These atoms are so unstable that they cannot exist alone. Therefore, two O atoms make bonds and create O2. This is a perfect example of this type of element. You will see the same for nitrogen too.
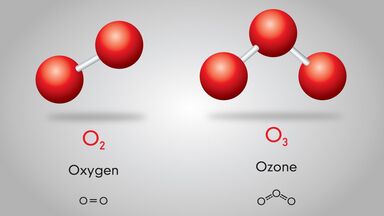
Diatomic Elements Occurrence
Hundreds of compounds have been discovered in the Earth’s environment. We also find them in the laboratory, and in interstellar space. The Earth’s atmosphere consists of two of these molecules. These are nitrogen (78 %) and oxygen (29 %). However, in the Earth’s atmosphere, the natural abundance of hydrogen (H2) is on the order of parts per million. As a result, H2 is the most abundant among them.
Diatomic Elements Meaning
In the 19th century, these elements were very much important in the development of the concepts of element, atom, and molecule. It is because we find some of the most common elements, as diatomic (e.g. such as hydrogen, oxygen, and nitrogen). John Dalton came up with the initial atomic hypothesis. He had assumed that all elements had only one atom. He also assumed that atoms in compounds have the simplest atomic ratios among themselves. And it is true in most cases. For example, Dalton assumed that water had the formula HO. It meant that the atomic weight of oxygen was eight times that of hydrogen. However, the actual value is roughly 16. As a result, there was a misunderstanding regarding atomic weights and molecular formulas. It continued for almost half a century.
In 1805, Gay-Lussac and von Humboldt demonstrated that water is two volumes of hydrogen and one volume of oxygen. By 1811, Amedeo Avogadro had successfully arrived at the proper chemical analysis of water. Based on these stands the now called Avogadro’s law. And also, the assumption of diatomic elemental molecules. However, these results were mostly ignored until 1860. Partly because it was thought that atoms of one element would have no chemical affinity for atoms of the same element. Also, because of apparent exceptions to Avogadro’s law were not explained until later.
Cannizzaro revived Avogadro’s ideas and used them to create a database of atomic weights. These weights were a necessary factor in the discovery of the periodic law by Dmitri Mendeleev and Lothar Meyer.
Diatomic Element Nomenclature
The naming system for these elements is somewhat lacking. Oxygen is the letter O on the periodic table. However, oxygen is also the gas that helps us to stay alive. Even if the air has O2, you will never find O atoms floating around in the environment. On the periodic table, the element oxygen is shown by the letter O. However, the oxygen present in the atmosphere is shown by the letter O2.
The logic here is that O is an oxygen atom. But the atoms live in pairs to make O2 oxygen gas. So, O atoms are just O, and oxygen gas means O2. Still, most of the time people just say “oxygen”. If they are talking about atoms or the periodic table, they probably mean O. But if they are talking about an actual substance they probably mean O2. It could go either way. However, based on the context, it’s usually pretty obvious which is which.
There is also a lot of nitrogen in the atmosphere. Though it shows N on the periodic table, in the atmosphere it is N2. Similarly, even though the periodic table only lists H, a canister of hydrogen gas would contain H2. When they put chlorine in the pool, it’s Cl2 (not Cl). And this is the same for all the seven elements.
Diatomic Elements Structure
The diatomic elements are elements that appear as molecules. In this type of element, there is a pair of atoms with a chemical bond. In the image below you can see the dot structures for F2, Cl2, Br2, I2, and H2.
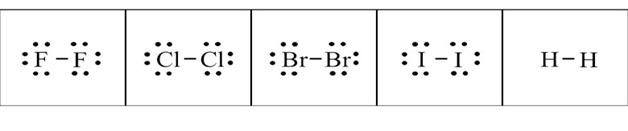
The horizontal line represents a bond between the pair of atoms. It is shown in letters. Now, why are the atoms more stable when they are in pairs? It is because they obey the octet rule. Let us discuss a bit about what this rule is.
The octet rule refers to the tendency of atoms to prefer to have eight electrons in the outermost shell. Atoms will react to get in the most stable state possible. A complete octet is very stable. Because all the shells will be full. Atoms with greater stability have less energy. So, increasing atoms’ stability releases energy in the form of heat or light.
The elements oxygen and nitrogen have a bit more complex structure. There is a double bond between the two O atoms in the O2 dot structure. And there is a triple bond between the two N atoms in the N2 dot structure.
Diatomic Elements in Periodic Table
Compounds consisting of two atoms that can exist as pure elements are part of this element group. All elements in this exclusive group are gases. Every molecule has its molecular formula. The formulas of these elements usually have a subscript of 2. It denotes the presence of two atoms in the structure. Consider the molecular formula to determine if you’re working with these elements. For example, the element oxygen has the formula O2. It indicates that there are two distinct oxygen atoms present.
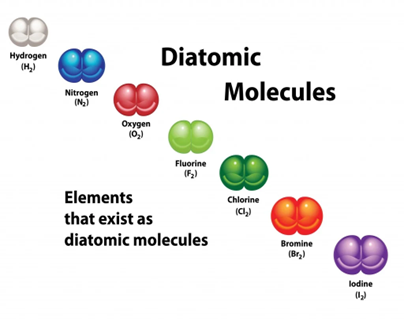
There are seven of these elements in the periodic table. Following is the list.
- Hydrogen (H2)
- Nitrogen (N2)
- Oxygen (O2)
- Fluorine (F2)
- Chlorine (Cl2)
- Iodine (I2)
- Bromine (Br2)

All of these elements are nonmetals since halogens are a special type of nonmetallic element. Bromine is a liquid at room temperature. While under ordinary conditions, the other elements are all gases. However, at lower temperatures or high pressure, the other elements become liquids.
Astatine (atomic number 85, symbol At) and tennessine (atomic number 117, symbol Ts) are also in the halogen group and may form these molecules.
It’s not that only these seven elements routinely form these molecules. Other elements can also form them. However, molecules formed by other elements are not very stable. Therefore, their bonds can easily break.
At room temperature five of these elements exist in the gas form. These are hydrogen, nitrogen, oxygen, fluorine, and chlorine. Two other elements bromine and iodine exist at high temperatures. At room temperature, bromine and iodine commonly exist in the liquid form. However, with that higher temperature, they will also exist as gases.
Diatomic Elements Mnemonics
Now, how do you memorize the elements quickly? There are several techniques to remember these elements. First, remember there are some common factors between these elements.
- Firstly, all of these elements are non-metal (mostly gases). None of the metal elements fall in this group.
- Secondly, the non-halogen ones all end with ‘-gen’. The halogen group also ends with ‘-gen’
Now, take a look at some mnemonics to help you quickly remember the elements. An easy-to-remember mnemonic for the elements is: Have No Fear of Ice-Cold Beer.
/what-are-the-seven-diatomic-elements-606623-v3-5b562dab46e0fb0037fee8c7.png)
Another great way to remember which atoms form the lucky seven elements is: I Bring Cookies for Our New Home. In this sentence, the first letter of each word helps you to remember one element.

Now, how do you identify these elements on the periodic table? You can easily do this if you remember ‘the seven’ rule. See the picture below. Now, starting at nitrogen, take your finger and draw the number seven. You will see that six of the elements are in the shape of a number seven. However, the seventh element is not part of this. It is the first one of the periodic table, hydrogen.

These elements are special. It is because atoms that form them do not like to be alone. Therefore, you will never find solo nitrogen or fluorine atom. Rather, these atoms will always pair together.
Diatomic Elements Acronym
The diatomic elements are hydrogen, oxygen, nitrogen, chlorine, bromine, iodine, and fluorine. One way of remembering the diatomic elements is by using mnemonics. We discussed this in the previous point. But what is another way to remember? Take a look at the acronym HONClBrIF. It is pronounced as honk-le-brif. This acronym includes the elemental symbol for each of the diatomic elements.

Diatomic Element and Lewis Structure
Some elements are more stable when they are with atoms of the same type. So they “prefer” to stay attached to another atom of the same element. Individual atoms are quite reactive. It is because they have incomplete valence shells and they are close to their correspondent noble gases. We can say that those atoms want to complete their shells. Therefore, we can explain this easily by the Lewis structure or Lewis Dot Formula.
Lewis structures show the bonding between atoms of a molecule. It can also be of the lone pairs of electrons that may exist in the molecule. You can draw Lewis Structure for any molecule, that has a covalent bond. You can also draw this for coordination compounds. The Lewis structure was named after Gilbert N. Lewis. He first introduced the concept in his 1916 article named, ” The Atom and the Molecule”. It follows the concept of the electron dot diagram. Atoms that represent shared pairs in a chemical bond are added using lines.
His structures show each atom and its position in the structure of the molecule. One can draw lines or use pairs of dots between atoms that are bonded to one another. Therefore, excess electrons that form lone pairs are represented as pairs of dots and are placed next to the atoms.
Are Diatomic Elements Compounds?
Now, the question arises of whether these elements are compounds or not? All the compounds are molecules, but molecules are not all compounds. This type of molecule is made up of only two atoms of the same or distinct elements. However, you may not find a chemical compound in every one of them.
Conclusion
We are at the end of our discussion. So, by now you have a clear idea about this type of element. Now, let us draw a brief conclusion on the whole topic. It will help us to understand the concept more clearly. So, diatomic elements are molecules with only two atoms. They can be of the same or different chemical elements. The prefix di- is a Greek word. It means “two”. If a molecule has two atoms of the same element, such as hydrogen (H2) or oxygen (O2), We call them homonuclear. On the other hand, if it is made up of two different atoms, such as carbon monoxide (CO) or nitric oxide (NO). Therefore, we call them heteronuclear.
These elements played an important role in explaining the concepts of atom and molecule in the 19th century. John Dalton’s original atomic hypothesis assumed that all elements were monoatomic. It means they had only one atom. Dalton assumed water’s formula to be HO. He took the atomic weight of oxygen eight times of hydrogen. Even if the modern value is about 16. Therefore, there was confusion about atomic weights and molecular formulas for about half a century.
F.A.Qs
What Are Diatomic Elements?
These are molecules with two atoms. However, the atoms can be of the same or different chemical elements.
What Are The 7 Diatomic Elements?
These are hydrogen (H, element 1), nitrogen (N, element 7), oxygen (O, element 8), fluorine (F, element 9), chlorine (Cl, element 17), bromine (Br, element 35), and iodine (I, element 53).
Are Diatomic Elements Compounds?
Though all compounds are molecules, not all molecules are compounds. Diatomic elements are molecules with only two atoms. Now they can be of the same or different elements.
How Many Diatomic Elements Are There?
There Are seven elements and they exist in their gaseous states. These are elements like Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, Bromine, And Iodine.
Is HCL A Diatomic Molecule?
All Diatomic Molecules are chemical compounds of two different elements. However, many elements can combine to form Heteronuclear Diatomic Molecules. It depends on factors like temperature and pressure. Hydrogen Chloride (HCl) is one of them.