Acid base reaction Calculator is a free online tool for calculating the equilibrium constant of a particular chemical reaction. Any online Acid base reaction calculator tool speeds up and simplifies the computation, displaying the equilibrium constant in a matter of seconds.

Moreover, a chemical reaction is a process. Moreover, it converts one or more reactants into one or more products. Then, chemical compounds or chemical elements used to describe the material. Then, the component atoms of the reactants rearrange to form distinct products throughout this process. Rust, for example, formed when iron and oxygen mix. In this article, we are talking about this calculator. So, keep reading to know more about it.
Acid base reaction calculator step by step
The following is the technique for using the chemical reaction calculator:
- First, enter the chemical reaction in the input area.
- Then, to obtain the results, click the “Submit” button.
- Atlast, the equilibrium constant for the selected chemical process will be shown in a new window.
Acid base reaction calculator chemistry
Moreover, the exchange of one or more hydrogen ions, H+, between species that may be neutral (molecules such as water, H2O; or acetic acid, CH3CO2H) or electrically charged (ions such as ammonium, NH4+; hydroxide, OH; or carbonate, CO32) is characterised by an acid–base reaction. Then, it also includes the behaviour of acidic molecules and ions that do not give hydrogen ions (aluminium chloride, AlCl3, and the silver ion AG+).
Moreover, acids are chemical substances that have a strong taste in water, a corrosive effect on metals, and the capacity to turn some blue vegetable colours red. Then, bases are chemical substances that are soapy to the touch in solution and convert red vegetable colours blue.
When acids and bases combine, they neutralize each other and form salts, which have a salty flavour but none of the qualities of either acid or base.
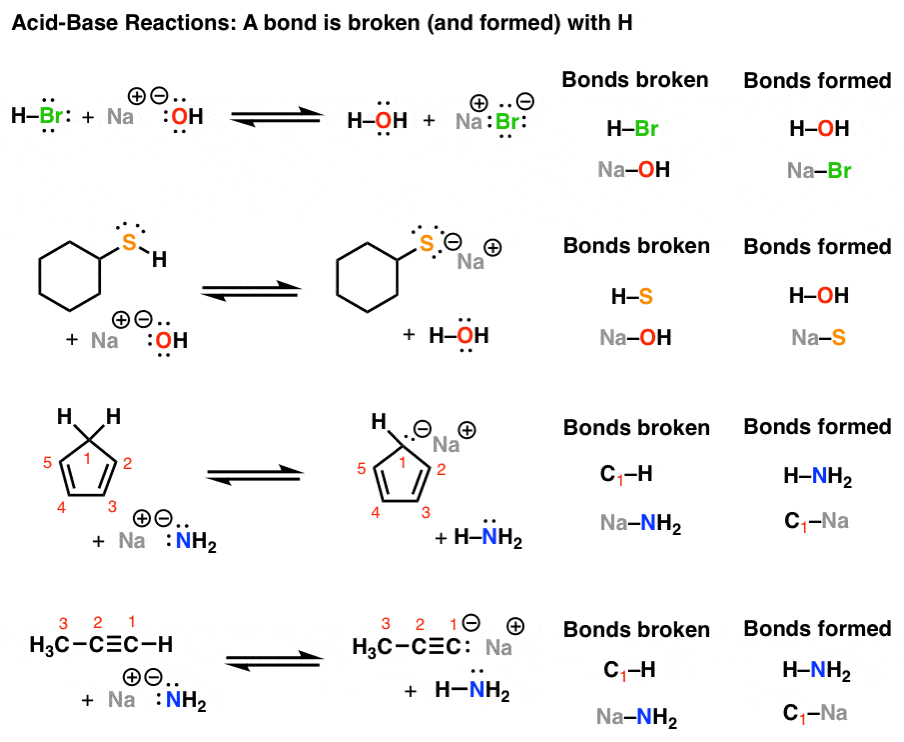
Then, the concept that certain substances are acids and others are bases is nearly as ancient as chemistry, and the phrases acid, base, and salt appear early in the works of mediaeval alchemists. Then, acids were most likely the first to be identified, owing to their sour taste. Also, the English term acid, French acide, German Säure, and Russian kislota are all derived from sour words (Latin acidus, German sauer, Old Norse sur, and Russian kisly).Read Also: Coterminal Angle Calculator Formula, Solved Examples, Step by Step
Salt
Moreover, other early features connected with acids were their solvent or corrosive activity, their influence on vegetable colours, and the effervescence produced when they were applied to chalk (production of bubbles of carbon dioxide gas). Then, bases (or alkalies) were distinguished primarily by their capacity to neutralize acids and create salts, the latter being defined quite vaguely as crystalline compounds that were soluble in water and had a salty taste.
Acid base reaction calculator organic chemistry
Proton transfers
Moreover, the Brnsted–Lowry definition described the reaction. Also, A B + H+, does not occur in any solution processes, as previously stated (The Bronsted–Lowry definition). Then, this is due to the fact that H+, the naked proton, has a strong proclivity to add to practically all chemical species. Also, it cannot exist in measurable amounts unless in a high vacuum.
Aside from any specific chemical interaction, the proton’s incredibly tiny size (approximately 1015 metres) implies that it creates an extraordinarily intense electric field. Also, it will polarise and hence attract any molecule or ion with which it comes into contact. Then, the dissociation of 19 grams of the hydronium ion H3O+ to yield 1 gramme of protons and 18 grams of water, predicted to need around 1,200,000 joules (290,000 calories) of energy, making it an exceedingly implausible operation.

Typical acid–base reactions are the combination of two reaction schemes, A1 B1 + H+ and H+ + B2 A2, resulting in the combined form A1 + B2 B1 + A2. This is a proton-transfer process from A1 to B2, resulting in B1 and A2. This method may be used to depict a huge number of reactions in solution, which are commonly referred to by a variety of names.
Acid base neutralization reaction calculator
Calculator
- Determine your substance’s equivalent weight. The equivalent weight of sodium chloride (NaCl), for example, is 58.443 g/eq.
- Decide how much of your ingredient you wish to neutralize. Assume you have 3 g of sodium chloride on hand.
- Determine the amount of solvent to be used (liquid). We’ll suppose you’d like to dissolve your NaCl sample in 0.1 litres of water.
- Use the following normality equation:
Normality = solute mass / (volume of solvent * equivalent weight)
3 g / (0.1 L * 58.443 g/eq) = 0.5133 eq/L = 0.5133 N
Molecular acid dissociation in water
Water serves as a basis in this case. For example, the equation for acetic acid dissociation is CH3CO2H + H2O ⇄CH3CO2 + H3O+.
Base dissociation in water
Moreover, the water molecule behaves as an acid in this example, adding a proton to the base. Then, H2O + NH3 ⇄OH + NH4+ is an example utilising ammonia as the base. Then, older formulations would have expressed the left-hand side of the equation as ammonium hydroxide, NH4OH. But this species is no longer thought to exist, save as a weak, hydrogen-bonded complex.
Acid-base dissociation in nonaqueous solvents
Moreover, these scenarios are completely equivalent to the corresponding reactions in water. Then, the dissociation of acetic acid in methanol, for example, represented as CH3CO2H + CH3OH ⇄CH3CO2 + CH3OH, and the dissociation of ammonia in the same solvent as CH3OH + NH3 ⇄CH3O + NH4+.
Amphoteric solvent self-dissociation
In this scenario, one solvent molecule serves as an acid, while another serves as a base. As examples, consider the self-dissociation of water and liquid ammonia:
H2O +H20 ⇄OH^- +H30^-
NH3 +NH3 ⇄NH2- +NH3+
Neutralization
Then, the neutralization process in water for a strong acid and a strong base occurs between hydrogen and hydroxide ions, i.e., H3O+ + OH ⇄2H2O. Then, for a weak acid and a weak base, neutralization should be thought of as a straight proton transfer from the acid to the base. Also, the neutralization of acetic acid by ammonia, for example, can be represented as CH3CO2H + NH3→ CH3CO2 + NH4+.
(124).jpg)
Because this equation does not include the solvent, it also reflects the process of neutralization in an inert solvent, such as benzene, or in the absence of a solvent entirely. (If one of the reactants is present in significant quantities, the reaction is more correctly characterized as acetic acid dissociation in liquid ammonia or ammonia dissociation in glacial acetic acid.)
Solution
How much 0.075 M HCl is needed to neutralize 100 mL of 0.01 M Ca(OH)2 solution?
So, HCl is a powerful acid that totally dissociates in water to H+ and Cl-. There will be one mole of H+ for every mole of HCl. The concentration of H+ will be 0.075 M since the concentration of HCl is 0.075 M.
Moreover, Ca(OH)2 is a strong base that entirely dissociates in water to Ca2+ and OH-. There will be two moles of OH- for every mole of Ca(OH)2. Because Ca(OH)2 has a concentration of 0.01 M, [OH-] will have a concentration of 0.02 M.
When the number of moles of H+ matches the number of moles of OH-, the solution is neutralized.
Acid base reaction calculator ph
- At first, fill a burette halfway with the titrant solution. Moreover, make careful not to pour the solution above your head, and that you remove the funnel once you’ve finished. Then, position the burette on a burette stand. Then, take note of the solution’s starting position on the burette. Also, to get to where the measurements begin, you may need to remove part of the solution.
- Then, add a quantity of the analyte to an Erlenmeyer flask (it should be less than the amount in your burette). Then, fill the flask with the indicator. Also, to best see the color, place it on a white tile beneath the burette.
- Then, begin carefully adding the titrant, stirring the Erlenmeyer flask frequently. Also, when the color change becomes too sluggish, begin adding the titrant drop by drop. Then, stop applying the solution after the color change is permanent.
- Then, take note of the burette’s terminus. Also, the difference between this and the beginning point provides you the volume, which you can use to compute the analyte’s molarity using the equation above.
- Then, all chemicals should be disposed of securely.
Methods
Moreover, titrations have several current applications, albeit many of the original purposes have been rendered obsolete by more recent techniques:
- First, to assist in determining the identity of an unknown solution, such as one gathered in the field. Although precise measurement is unattainable. So, titration is a valuable technique for determining molarity. Also, we can use the titration curve to identify whether the solution is a strong or weak acid/base.
- If waste vegetable oil is used to make biodiesel, the batch must be neutralized before processing. Then, to do this, a tiny sample titrated to determine its acidity, which tells us how much base we need to effectively neutralize the batch. Then, the use of a base eliminates the current free fatty acids.
- Moreover, we frequently used titrations to determine the amount of acid rain that falls. So, we can use these tests to track the [level of pollution](calc:1210) in the high atmosphere.
Acid base reaction calculator equation
A strong acid-strong base reaction is essentially a quantitative reaction. As an example,
HCl(aq) + Na(OH)(aq) → H2O + NaCl(aq)
As part of the neutralization reaction, both sodium and chloride ions are present in this reaction.
H+ + OH− → H2O
Does not involve them. Because a weak base solution is a buffer solution, the addition of acid is not quantitative. Moreover, a buffer solution is also a weak acid solution. An equilibrium mixture is formed when a weak acid combines with a weak base. Adenine, denoted as AH, can, for example, combine with a hydrogen phosphate ion, HPO2-4.
AH + HPO2−4 ⇌ A− + H2PO−4
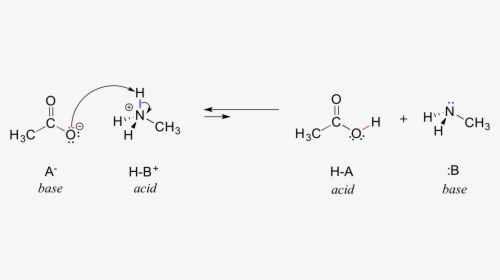
The acid dissociation constants of adenine and the di-hydrogen phosphate ion may be used to calculate the equilibrium constant for this process.
[A−] [H+] = Ka1[AH]
[HPO2−4] [H+] = Ka2[H2PO−4]
The symbol [X] stands for “concentration of X”. After combining these two equations, a formula for the equilibrium constant, K, produced by removing the hydrogen ion concentration.
[A−] [H2PO−4] = K[AH] [HPO2−4]; K = Ka1/Ka2.
Acid base reaction calculator products
By matching each cation with the anion of the opposite chemical, you may anticipate the results of acid-base reactions.
Acid-base neutralization is a subset of the double displacement process. We can forecast the goods using the same strategies. The cation in acids is always H+.
As an example, consider the neutralization of stomach acid (HCl) by milk of magnesia [Mg(OH)2].
Mg(OH)2 + HCl →?
(a.) Separate the acid into H + ions and negative ions.
HCl→H^+ +Cl^-
(b.) Separate the base into positive and OH – ions.
Mg(OH)2→Mg2 +OH-
(c.) For the products, mix the H + and OH – ions to form H2O.
The positive and negative ions, then combined to form a salt. Remember to keep the salt’s positive and negative charges balanced.
In this case,
Mg2+ and Cl- make MgCl2.
(H+ +Cl-)+(Mg2+ +OH-) →(Mg2+ +Cl-)+(H+ +OH-)
HCl + Mg(OH)2→MgCl2+H2O
(d.) Balance the equation.
2HCl+Mg(OH)2→MgCl2+2H2O
Compile and balance an equation for the reaction of HBr and Al(OH) 3.
Solution
HBr+Al(OH)3→?
(H+ +Br-)+(Al3+ +OH-)→(Al3++Br-)+(H+ +OH-)
HBr + Al(OH)3→AlBr3+H2O
3HBr + Al(OH)3→AlBr3+3H2O
Acid base reaction calculator balance
- First, enter a chemical reaction equation and press the ‘Balance’ button. Then, the solution will be provided below.
- Then, the initial character in the element name should always be capitalized, and the second character should be lowercase. Also, Fe, Au, Co, Br, C, O, N, F is a few examples. Co – cobalt and CO – carbon monoxide are comparable terms.
- Then, use – or e to input an electron into a chemical equation.
- Then, to enter an ion, enter the charge after the chemical in curly brackets: {+3} or {3+} or {3}.
- Also, for instance, Fe{3+} + I{-} = Fe{2+} + I2
- Then, to prevent uncertainty, substitute immutable groups in chemical compounds.
- Then, the equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O, for example, will not be balanced.
- However, PhC2H5 + O2 = PhOH + CO2 + H2O will work.
- Then, the use of compound states [such as (s) (aq) or (g)] is not necessary.
- If you don’t know what products are, just input reagents and hit the ‘Balance’ button. In many circumstances, an entire equation will be proposed.
- A balanced equation’s reaction stoichiometry might be determined. To calculate the remainder, enter either the number of moles or the weight of one of the compounds.
- For a balanced equation, the limiting reagent may be calculated by inputting the total number of moles or weight of all reagents. The row containing the limiting reagent will be highlighted in pink.
Examples
- Fe + Cl2 = FeCl3
- KMnO4 + HCl = KCl + MnCl2 + H2O + Cl2
- K4Fe(CN)6 + H2SO4 + H2O = K2SO4 + FeSO4 + (NH4)2SO4 + CO
- C6H5COOH + O2 = CO2 + H2O
- K4Fe(CN)6 + KMnO4 + H2SO4 = KHSO4 + Fe2(SO4)3 + MnSO4 + HNO3 + CO2 + H2O
- Cr2O7{-2} + H{+} + {-} = Cr{+3} + H2O
- S{-2} + I2 = I{-} + S
- PhCH3 + KMnO4 + H2SO4 = PhCOOH + K2SO4 + MnSO4 + H2O
- CuSO4*5H2O = CuSO4 + H2O
- calcium hydroxide + carbon dioxide = calcium carbonate + water
- Sulphur + ozone = sulphur dioxide
Acid base reaction calculator acidity constants
The table includes acidity constants for several substances. These are acids or bases. Acids or bases, based on the nature of the uncharged species. However the value provided in each case is pKa for the acid form (pKa and pKb for a conjugate acid–base pair linked using the equation for aqueous solutions at 25 °C). When various pKa values are given, they refer to sequential dissociations; for example, for phosphoric acid, they correspond to dissociations of H3PO4, H2PO4-, and HPO42-. All values given are for aqueous solutions at or around 25 °C; parentheses indicate indirect estimates or values that are questionable for other reasons.
Acid base reaction calculator examples
Example 1
How to calculate the Molarity of a percentage solution?
As an example, using 70% concentrated nitric acid: The term “70% nitric acid” refers to the fact that 100 grams of this acid contain 70 grams of HNO3. The concentration is given as 70 percent wt/wt or 70 percent HNO3. Some chemists and analysts prefer to operate with Molarity (moles/litre) units of acid concentration. The number of moles of HNO3 present in 1 litre of acid must be calculated to determine the molarity of a 70% wt. nitric acid. Knowing the acid’s density of 1.413 g/mL, we can compute the weight of 1 L of 70% HNO3 to be 1413 grams.

Knowing that the solution contains 70% HNO3, the number of grams of HNO3 per litre may be calculated: (0.700)(1413g) = 989.1 grams HNO3 per litre. The number of moles of HNO3 / L or Molarity is 15.7 M when the molecular weight of HNO3 (63.01 g/mole) divides the grams of HNO3.
Example 2
How do I calculate the normality of an acid or base from its molarity?
When the concentration is given in weight percent, the following equation is used to calculate acid and base molarity:
[(percentage of d) / MW] Molarity = 10
Where: % = Weight Percentage; d = Density (or Specific Gravity); and MW = Molecular Weight (or Formula Weight).
The aforementioned equation may then be used to compute the molarity of the 70% Nitric Acid:
[(701.413) / 63.01] 10 x 15.7 M.
Some frequently asked questions
What is an example of an acid-base reaction?
To chemists, a salt is a result of an acid-base reaction that consists of the cation from the base and the anion from the acid. We can see the creation of salt and water, when hydrochloric acid combines with sodium hydroxide. Na+ cations from the base (NaOH) and Cl anions from the acid combine to form sodium chloride (HCl).
What is the equation for an acid-base reaction?
After combining a strong acid and a strong base, they react according to the net-ionic equation: H3O+(aq) + OH(aq)→ 2H2O (l). If either the acid or the base is present in excess, the pH of the resultant solution may be calculated using the excess reactant concentration.
What is acid or base?
Any hydrogen-containing material capable of transferring a proton (hydrogen ion) to another chemical is an acid. A base is a molecule or ion that can receive hydrogen ions from acids. We used the sour taste of acidic chemicals to identify them.
What is the role of acid in our stomach?
Stomach acid, also popular as gastric acid, is a colorless, watery fluid. The lining of the stomach generated it. It is very acidic and aids digestion by breaking down food. This makes it easier for your body to absorb nutrients when food passes through your digestive tract.
What are acid bases and salt?
We can see the creation of a salt, after replacing hydrogen ions using a metal or an ammonium ion in an acid. A base is a material that interacts with an acid to produce solely salt and water. We can see the creation of salt, when an acid interacts with a base.
What is the formula of acid?
| Compound | Formula | Specific Gravity |
| Acetic acid, glacial | CH3COOH | 1.05 |
| Formic acid | HCOOH | 1.20 |
| Hydrochloric acid | HCl | 1.18 |
| Nitric acid | HNO3 | 1.42 |
What do bases do?
In chemistry, a base is any material that is slippery to the touch in aqueous solution, tastes bitter, alters the color of indicators (e.g., turns red litmus paper blue), interacts with acids to generate salts, and stimulates specific chemical processes (base catalysis).
What are 5 examples of bases?
- (KOH)
- (NaOH)
- (Ba(OH)2)
- (CsOH)
- (Sr(OH)2)
What is an acid base reaction calculator?
Chemical Process Calculator is a free online tool for calculating the equilibrium constant of a particular chemical reaction. Any online chemical reaction calculator tool speeds up and simplifies the computation, displaying the equilibrium constant in a matter of seconds.