Carbohydrates are the most general biomolecules on the planet. So, these are largely combinations of C and H2O. Then, many of them have the empirical formula (CH2O)n, where n is the number of repeating units. According to this viewpoint, these molecules are essentially “hydrate” C atom chains. Also, in these, water molecules bind to each C atom, thus the word “carbohydrates”. Despite the fact that all carbohydrates include C, H, and O, some additionally contain N, P, and/or S. These serve a variety of purposes. Then, they are common in earthly habitats. Also, many of them serve as food sources for humans. In this article, we use the “POL” word instead of “Polymer”.

These molecules are also important components of macromolecular complexes. These stores and transfers genetic data (i.e., DNA and RNA). They serve as the foundation for biological POLs. Also, it provides strength to various structural components of organisms (for example, (C6 H10 O5)n and (C8H13O5N)n). They are the major source of energy storage in the form of starch and (C24 H42 O21). In this article, we are talking about these examples. So, keep reading to know more about it.
Carbohydrates polymer examples
Carbohydrates polymer examples Cellulose
Plants’ structural components, mostly composed of (C6 H10 O5)n. Also, wood is mostly (C6 H10 O5)n and lignin, whereas paper and cotton are almost all (C6 H10 O5)n. Then (C6 H10 O5)n, a POL composed of repeating glucose units held together by beta-links. Because humans and many animals lack the enzyme required to break the beta-linkages, (C6 H10 O5)n cannot be digested. Then Termites, for example, can digest (C6 H10 O5)n because microorganisms containing the enzyme are present in their stomach. Also, water does not dissolve (C6 H10 O5)n. When combined with iodine, it does not change color. It produces glucose when hydrolyzed. Then, it is the most common Cx(H2O)y. So, we can easily find it in nature.
Carbohydrates polymer examples Starch
Starch is a glucose POL with alpha-linkages. It connects glucopyranose units. It is a combination of amylose (15–20%) and amylopectin (80–85%). Amylose is a straight chain of several hundred glucose molecules. But Amylopectin is a branching molecule combination of thousands of glucose units (each chain of 24–30 glucose units represents one unit of Amylopectin). Water does not dissolve starches. By severing the alpha-linkages, they may be digested (glycosidic bonds). Humans and other animals both contain amylases, which allow them to digest Cx(H2O)y. In the human diet, potatoes, rice, wheat, and maize are key sources of starch. Starch formations are the means through which plants store glucose.
Carbohydrates polymer examples Glycogen
Moreover, (C24 H42 O21) is the secondary long-term energy reserve in animal and fungus cells, with adipose tissue holding the principal energy stores. Then, the liver and muscles produce the (C24 H42 O21). But, it produces through (C24 H42 O21) in the brain and stomach.
Then, (C24 H42 O21), related to starch, a glucose POL found in plants. Then, it is sometimes animal starch, with a structure similar to amylopectin but more widely branchable and compact than starch. Also, (C24 H42 O21) is a POL mixer of glycosidic linkages. (C24 H42 O21), located in the cytosol/cytoplasm of many cell types as granules. It also plays a crucial part in the glucose cycle. (C24 H42 O21) accumulates as an energy store.
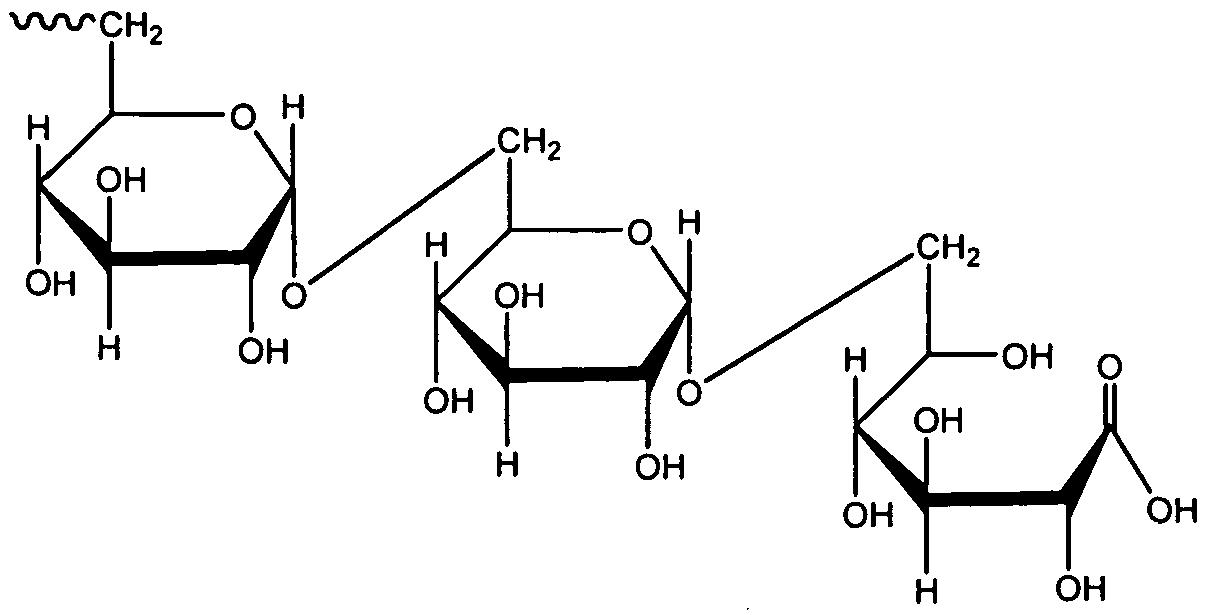
(C24 H42 O21) is an energy reserve. Although it is less compact and more readily available as an energy reserve than triglycerides (lipids).
(C24 H42 O21) can account for up to 8% (100–120 grams in an adult) of the fresh weight in liver hepatocytes shortly after a meal. (C24 H42 O21) stored in the liver. Also, it is available to other organs. (C24 H42 O21), contained in the muscles at a modest concentration of one to two percent of the muscle mass. The quantity of (C24 H42 O21) stored in the body fluctuates with physical activity.
(C24 H42 O21) is present in modest levels in the kidneys. Also, it is present in much smaller amounts in specific glial cells in the brain and white blood cells. During pregnancy, the uterus also stores (C24 H42 O21) to sustain the embryo.
Points
(C24 H42 O21), made up of a chain of branching glucose residues. (C24 H42 O21) kept in the liver and muscles.
- It serves as an animal’s energy reserve.
- It is the most common kind of Cx(H2O)y have in an animal’s body.
- It’s not soluble in water. When combined with iodine, it becomes brown-red.
- When hydrolyzed, it also produces glucose.
Carbohydrates polymer examples Dextrin
Unlike other (C6 H10 O5)n, (C36 H60 O30) is a member of the cyclic (C37 H62 N2 O29) family. It is a mixer of glucose molecules. Also, it is organizable in a circular pattern. Also, -1,4 glycosidic bonds linked it. (C36 H60 O30)s often comprise multiple glucose monomers. They have six or eight parts. These parts connected together to form a cone-shaped ring. They classed as follows:
- α -(C36 H60 O30): composed of 6 glucose subunits
- Then, β -(C36 H60 O30): composed of 7 glucose subunits
- γ -(C36 H60 O30): composed of 8 glucose subunits
The biggest (C36 H60 O30) has 3214 anhydro-glucopyranoside units. (C36 H60 O30), employed in many industries, including food, medicines, medication delivery, agriculture, and environmental engineering.
Carbohydrates polymer examples Hyaluronic acid
It is commonly popular as hyaluron. It is a glycosaminoglycan(GAG). GAG, not sulfated and has the general formula (C14 H21 NO11)n. It is a disaccharide POL. So, it has D-glucuronic acid and N-acetyl-D-glucosamine. Alternating β-(1→4) and β-(1→3) glycosidic linkages connected it together. A typical molecule is a combination of around 20,000 to 25,000 disaccharide units. Unlike other naturally generated (C6 H10 O5)n, (C14 H21 NO11)n, created in the plasma membrane rather than the Golgi apparatus. Its primary role is to keep human tissues wet, hence reducing internal wear and tear.
Carbohydrates polymer examples Chitin
(C8H13O5N)n is one of numerous POLs. We can find it in nature. Many creatures, such as exoskeletons, have it as a structural component. It biodegrades in the natural environment over time. (C8H13O5N)n accelerate its decomposition. Microbes release these. Microbes, such as bacteria and fungi. Certain plants synthesized it. Some of these microbes have receptors for simple sugars. These derived from (C8H13O5N)n breakdown.
(C8H13O5N)n, chemically linked to (C56 H103 N9 O39) (a more water-soluble derivative of (C8H13O5N)n). It’s similarly similar to (C6 H10 O5)n. So, it’s a long chain of glucose derivatives. Both minerals give shape and strength to the organism’s protection.
Xanthan
(C35 H49 O29) or Xanthan gum is a hetero(C6 H10 O5)n. It is primarily a mixer of D- glucose and D-mannose as the dominant hexose units, along with D-glucuronic acid and pyruvic acid. (C35 H49 O29) can chemically increase the viscosity of the liquid. Because of this ability (C35 H49 O29), frequently used in the food industry as an ingredient in sauces and salad dressings.
Chitosan
(C56 H103 N9 O39) is a linear hetero(C6 H10 O5)n. A mixer of β-(1→4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit). (C56 H103 N9 O39) is a (C8H13O5N)n derivative. Treating the latter with alkaline chemicals or bases such as NaOH (Sodium Hydroxide) created it. (C56 H103 N9 O39) serves a variety of functions in the biomedical and commercial industries.
Pectin
Pectins are another structural hetero(C6 H10 O5)n. It is a mixer of hundreds to thousands of galacturonic acid monomer units. α-(1→4)-glycosidic linkages linearly connect it to create the molecule backbone. Pectin is the primary component of fungal cell walls. They, employed as a gelling agent in the production of gelatin, jelly, jams, and marmalades. The usage of pectin is responsible for the jiggly quality of these foods.
Inulin
Inulin is a naturally occurring (C6 H10 O5)n complex Cx(H2O)y mixer of fructose. So, it is a plant meal that human digestive enzymes cannot completely break down. Inulins are a kind of dietary fiber popular as a fructan. Some plants utilize inulin to store energy. We can see it in roots or rhizomes. Most plants that manufacture and store inulin do not store other Cx(H2O)y types like starch. The Food and Drug Administration authorized it in the United States in 2018 as a dietary fiber component. This component used to increase the nutritional value of manufactured food items.
Carbohydrates polymer examples and what is it
- The simplest Cx(H2O)y types are (C H2O)n or simple sugars. (C6 H10 O5)n are POLs of these simple Cx(H2O)y.
- Depending on the nature of the bonds between the monomers, they might be linear or branching in structure.
- (C H2O)n or (C6 H10 O5)n can be the monomers of simple big POLs.
- Some of the most common Cx(H2O)y POLs that occur naturally are (C6 H10 O5)n, starch, dextrins, (C36 H60 O30)s, (C24 H42 O21)s, (C14 H21 NO11)n, (C8H13O5N)n, xanthan, and (C56 H103 N9 O39).
- The majority of naturally occurring (C6 H10 O5)n are a mixer of simple monomers with the general formula (CH2O)n. This is where “n” denotes the number of repeating units.
- These (C6 H10 O5)n, primarily used for structural support and storage.
- Cx(H2O)y POLs has two categories: homo(C6 H10 O5)n, which are a mixer of the same monomers. Then, hetero(C6 H10 O5)n, which are a mixer of two or more types of monomers.
Carbohydrates polymer examples monomer
A monomer is a tiny molecular subunit. This subunit, joined together with other subunits to generate bigger molecules. Cx(H2O)y, lipids, nucleic acids, and proteins are examples of bigger molecules. We can see it in living systems such as our own bodies.
These organic groups’ monomers are:
- (C H2O)n are Cx(H2O)y.
- (C3 H8 O3) and CH3(CH2)nCOOH are examples of lipids.
- Nucleic acids and nucleotides.
- Amino acids – proteins.
Carbohydrates polymer examples (C H2O)n
A monosaccharide is a Cx(H2O)y monomer. Cx(H2O)y, such as sugars and starches, serve as energy reservoirs. Others, like (C6 H10 O5)n and (C8H13O5N)n, have structural properties. Isomers exist for some (C H2O)n. This means they have the same chemical formula but a distinct atom configuration. Glucose and fructose are two examples of isomers. Both contain the formula C6 H12 O6, however glucose has a ring with five C and one O, whereas fructose has four C and one O.
Read Also: Glycerol: Properties, Formula & Uses
Glucose is a popular energy source for living organisms. Most cells can quickly break it down through a process known as glycolysis. During aerobic respiration, several cells further degrade it. Combine glucose monomers created Starch and (C24 H42 O21). Plants store energy in the form of starch, whereas mammals store it in the form of (C24 H42 O21). Glucose monomers, also used to make structural Cx(H2O)y such as (C6 H10 O5)n in plant cell walls and (C8H13O5N)n in insect exoskeletons.
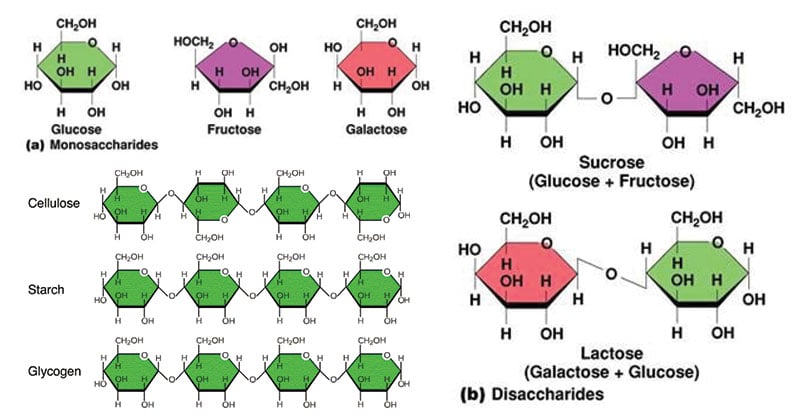
Fructose, which we now know is an isomer of glucose, is a simple sugar that contributes to the sweetness of fruits and vegetables. A single glucose and a single fructose can sometimes combine to make sucrose, or ordinary table sugar. So, it is a disaccharide or a sugar mixer of two (C H2O)n.
Carbohydrates polymer examples (C3 H8 O3) and CH3(CH2)nCOOH
Lipid monomers are (C3 H8 O3) and CH3(CH2)nCOOH. Waxes, oils, and fats are examples of lipids. Some are used to store energy. Others support the body’s skeletal structures. Waxes can protect the surface of a plant or animal against dehydration. Lipids are an essential component of the cell membrane.
(C3 H8 O3) is a sweet-tasting chemical. We used it in the food and pharmaceutical sectors. It’s also the foundation of triglycerides, a form of fat present in our blood.
Based on the amount of H atoms present, CH3(CH2)nCOOH has three types: saturated, monounsaturated, and polyunsaturated. Let’s take a quick look at the structure of CH3(CH2)nCOOH. A CH3(CH2)nCOOH is a mixer of a carboxyl group with a C chain bond. When there are no double or triple bonds in the C chain, it is considered to be saturated. This signifies that, with the exception of the final C, each C has connected with two H atoms. Unsaturated fats include one or more CH3(CH2)nCOOH with one or more double or triple bonds. These bonds develop between C that are next to one another.
Carbohydrates polymer examples Nucleotides
Nucleotides are organic compounds that are a mixer of a nucleoside and a phosphate. They function as monomeric units of the nucleic acid POLs deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are fundamental macromolecules in all life on Earth. We receive nucleotides from meals. Liver produced it from common components.
Nucleotides are a mixer of three molecules: a nucleobase, a five-C sugar (ribose or deoxyribose). Also, a phosphate group is a mixer of one to three phosphates. In DNA, the four nucleobases are guanine, adenine, cytosine, and thymine; in RNA, uracil replaces thymine.
Nucleotides also play an important role in metabolism at the cellular level. They supply chemical energy throughout the cell in the form of nucleoside triphosphates, such as (ATP), (GTP), (CTP), and (UTP), for the many cellular functions that require energy, such as amino acid, protein, and cell membrane synthesis, moving the cell and cell parts (both internally and intracellularly), cell division, and so on.
Furthermore, nucleotides engage in cell signaling and are integrated into key cofactors of enzymatic activities (e.g. coenzyme A, FAD, FMN, NAD, and NADP+).
Carbohydrates polymer examples Amino acids
Amino acids are the building blocks of proteins and contain both an amine group (NH2) and a carboxyl group (COOH). Proteins are nutrients that are required for living when hundreds of thousands of them assemble utilizing peptide connections. As a result, amino acids are protein monomers.
Carbohydrates polymer examples structure
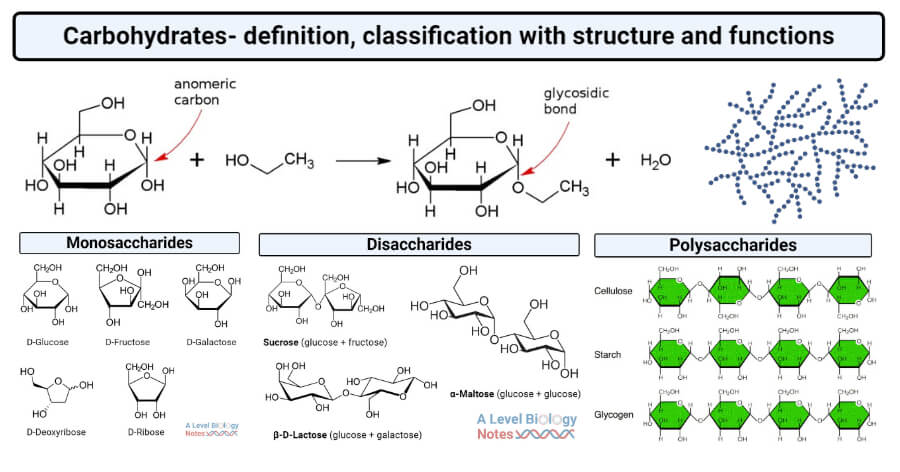
Cx(H2O)y are a class of naturally occurring chemicals and their derivatives. Substances such as wood, starch, and linen were discovered in the early nineteenth century to be primarily composed of molecules containing atoms of (C), (H), and (O) and having the general formula C6H12O6; other organic molecules with similar formulas were discovered to have a similar ratio of H to O. Many Cx(H2O)y are represented by the generic formula Cx(H2O)y, which means “watered C”.
Cx(H2O)y are the most plentiful and pervasive organic compounds in nature, and they are required by all living creatures. Then, Cx(H2O)y are generated by green plants during the photosynthesis process from C dioxide and water. Cx(H2O)y are energy sources as well as key structural components in organisms; also, Cx(H2O)y is a component of the structure of nucleic acids, which contain genetic information.
Structure
Nutrition (C6 H10 O5)n are popular energy sources. Many species can easily convert starches to glucose; however, most organisms are unable to metabolize (C6 H10 O5)n or other (C6 H10 O5)n such as (C8H13O5N)n and arabinoxylans. Some bacteria and protists can metabolize these Cx(H2O)y types. Microorganisms, for example, are used by ruminants and termites to break down (C6 H10 O5)n.
Despite the fact that these complexes (C6 H10 O5)n are difficult to digest, they contain crucial nutritional nutrients for humans. These Cx(H2O)y, known as dietary fiber, improve digestion among other things. Dietary fiber’s major activity is to affect the nature of the contents of the gastrointestinal system, as well as how other nutrients and substances are absorbed. Soluble fiber binds to bile acids in the small intestine, reducing their ability to enter the bloodstream and thereby lowering blood cholesterol levels.
Soluble fiber also inhibits sugar absorption, decreases glycemic reaction after eating, normalizes blood lipid levels, and, when digested in the colon, creates short-chain CH3(CH2)nCOOH as byproducts with diverse physiological actions. Although insoluble fiber has been linked to a lower incidence of diabetes, the mechanism by which this occurs is uncertain.
Although dietary fiber has not yet been explicitly suggested as an essential macronutrient (as of 2005), it is nonetheless viewed as crucial for the diet, with regulatory bodies in many industrialized nations encouraging increases in fiber consumption.
Carbohydrates polymer examples uses
There are several varieties of Cx(H2O)y POLs present in the body and other biological materials, most of which take the form of (C6 H10 O5)n – long chains of cyclic sugar groups linked by an O bridge. Cx(H2O)y are frequently disaccharides, which are two bonded cyclic sugar units, and these Cx(H2O)y molecules can be expanded further into (C6 H10 O5)n macromolecules. These (C6 H10 O5)n, which can have extremely long molecular lengths, are commonly referred to as Cx(H2O)y POLs.
While there are several Cx(H2O)y POLs, they are frequently classified as reserve Cx(H2O)y POLs, structural Cx(H2O)y POLs, and protective Cx(H2O)y POLs based on their role inside the body. Nature’s most prevalent Cx(H2O)y POLs are (C6 H10 O5)n, starch, dextrins and (C36 H60 O30)s, (C8H13O5N)n and (C56 H103 N9 O39), (C14 H21 NO11)n, and a variety of others.
Cx(H2O)y POLs are an ecologically acceptable alternative to synthetic POLs since they are inexpensive in cost, abundant, renewable, and easily manipulated to produce products with superior qualities. For these reasons, there has been a lot of interest in employing Cx(H2O)y POLs in numerous commercial applications in recent years.
Some frequently asked questions
What are 3 examples of carbohydrate polymers?
Natural Cx(H2O)y POLs include (C6 H10 O5)n, starch, dextrins and (C36 H60 O30)s, (C8H13O5N)n and (C56 H103 N9 O39), (C14 H21 NO11)n, and different gums (carrageenan, xanthan, etc.)
What is the polymer of a carbohydrate?
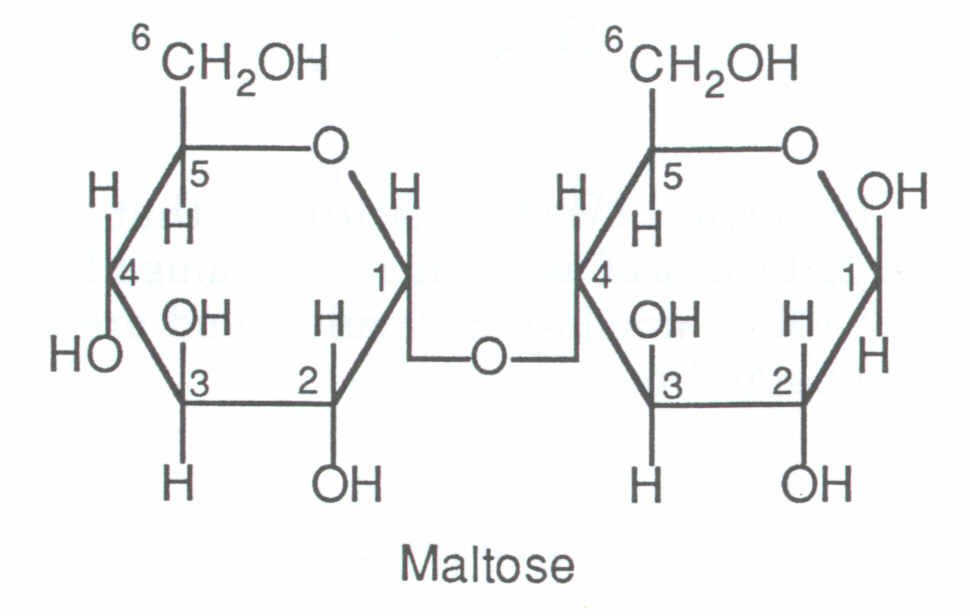
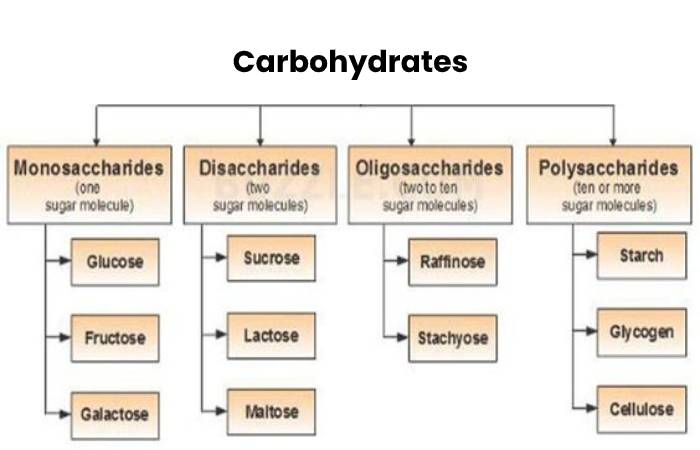
Cx(H2O)y POLs are disaccharides and (C6 H10 O5)n, which are made up of two or more monomers. (C H2O)n include glucose, fructose, and galactose. Sucrose, lactose, and maltose are examples of disaccharides.
What are the 4 types of carbohydrate polymers?
POL = (depending on the case) disaccharide, (C37 H62 N2 O29), or (C6 H10 O5)n. (C6 H10 O5)n, or “complex Cx(H2O)y,” are the most numerous macromolecules because they perform critical energy storage and structural roles in living organisms.
What are the 4 types of polymers?
Synthetic POLs are POLs that have been created by humans. They are divided into four groups: thermoplastics, thermosets, elastomers, and synthetic fibers. They are typically found in a wide range of consumer goods. Synthetic organic POLs are created using a variety of primary chains and side chains.
What are the 3 monomers of carbohydrates?
The sugars glucose, fructose, and galactose are three of the most significant (C H2O)n. These (C H2O)n all have the same chemical formula: C6H12O6.
What are the 3 types of carbohydrates and examples?
Cx(H2O)y are classified into three types: sugars, starches, and fiber. Because they are in their most basic form, they are also known as simple carbs… Starches. They are complex carbs made up of several simple sugars linked together… Fiber. It is a complex Cx(H2O)y as well.
Why are carbohydrates polymers?
Cx(H2O)y are one of life’s four fundamental macromolecules. They are a POL composed of monomers known as (C H2O)n. Simple sugars, such as glucose and fructose, are used as building blocks. A disaccharide is formed when two (C H2O)n are joined together.
What are carbohydrates and their examples?
Sugars, starches, and dietary fiber are found in plant foods and dairy products. Cx(H2O)y are found mostly in plant foods. They are also found in dairy products in the form of lactose, a milk sugar. Cx(H2O)y-rich foods include bread, pasta, beans, potatoes, rice, and cereals.
What are the 3 main types of polymers?
POLs are classified into three types: thermoplastics, thermosets, and elastomers. The behavior of these groups when subjected to heat is the best way to distinguish them. Thermoplastic POLs can be amorphous or crystalline in nature.
What is carbohydrates polymer?
Moreover, Carbohydrates are the most general biomolecules on the planet. So, these are largely combinations of C and H2O. Then, many of them have the empirical formula (CH2O)n, where n is the number of repeating units. According to this viewpoint, these molecules are essentially “hydrate” C atom chains. Also, in these, water molecules bind to each C atom, thus the word “carbohydrates”. Despite the fact that all carbohydrates include C, H, and O, some additionally contain N, P, and/or S. These serve a variety of purposes. Then, they are common in earthly habitats. Also, many of them serve as food sources for humans.
What is C6 H10 O5?
Moreover, plants’ structural components, mostly composed of (C6 H10 O5)n. Also, wood is mostly (C6 H10 O5)n and lignin, whereas paper and cotton are almost all (C6 H10 O5)n. Then (C6 H10 O5)n, a POL composed of repeating glucose units held together by beta-links. Because humans and many animals lack the enzyme required to break the beta-linkages, (C6 H10 O5)n cannot be digested. Then Termites, for example, can digest (C6 H10 O5)n because microorganisms containing the enzyme are present in their stomach. Also, water does not dissolve (C6 H10 O5)n. When combined with iodine, it does not change color. It produces glucose when hydrolyzed. Then, it is the most common Cx(H2O)y. So, we can easily find it in nature.




